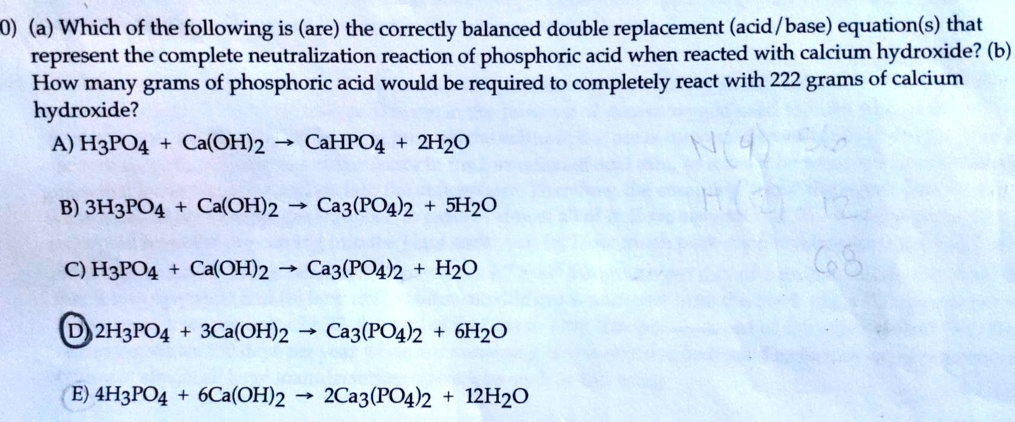
SOLVED: 0) (a) Which of the following is (are) the correctly balanced double replacement (acid /base) equation(s) that represent the complete neutralization reaction of phosphoric acid when reacted with calcium hydroxide? (b)

Acid and Base Chemistry. Some Properties of Acids þ Produce H + (as H 3 O + ) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) - ppt download
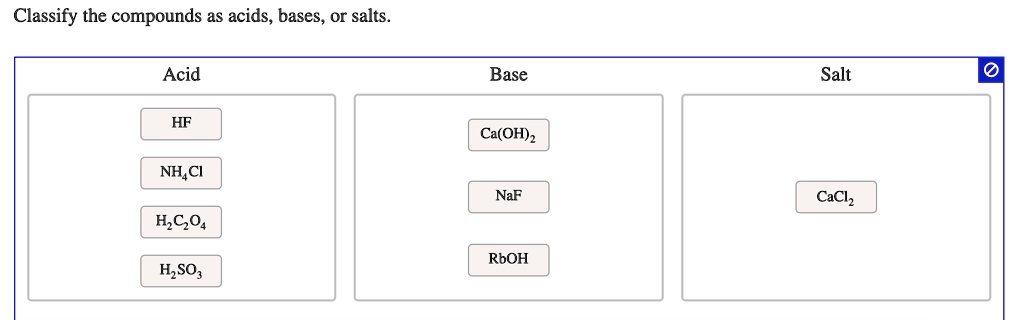

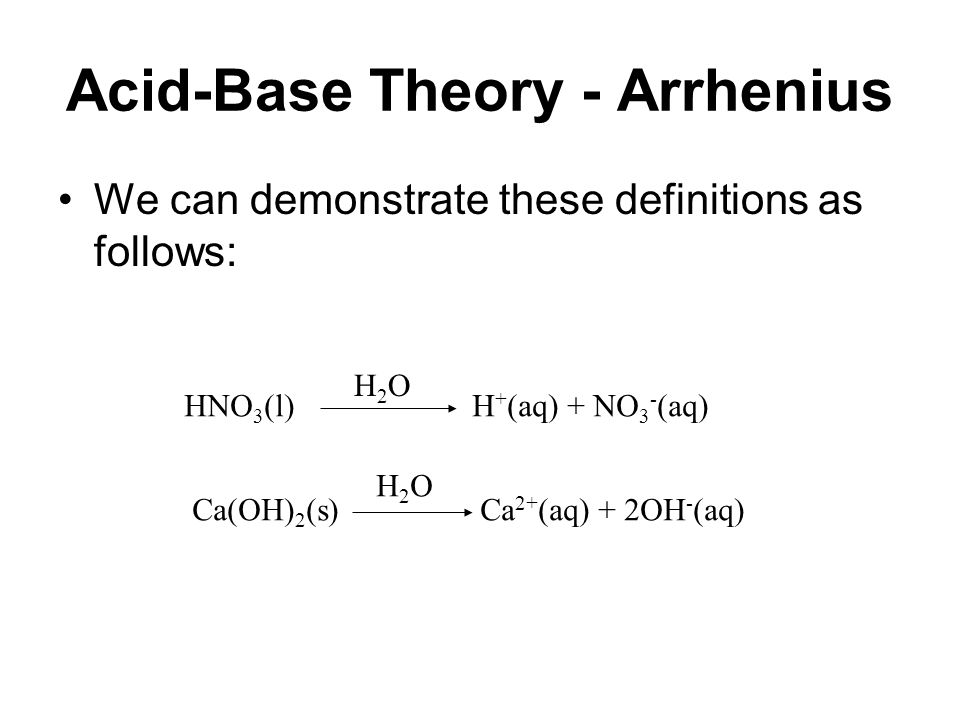





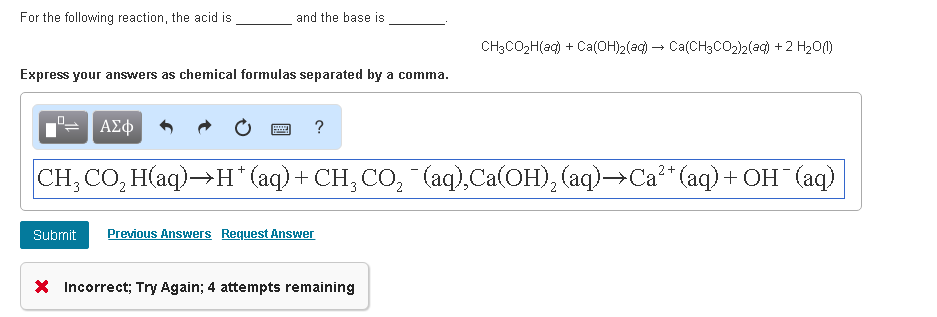

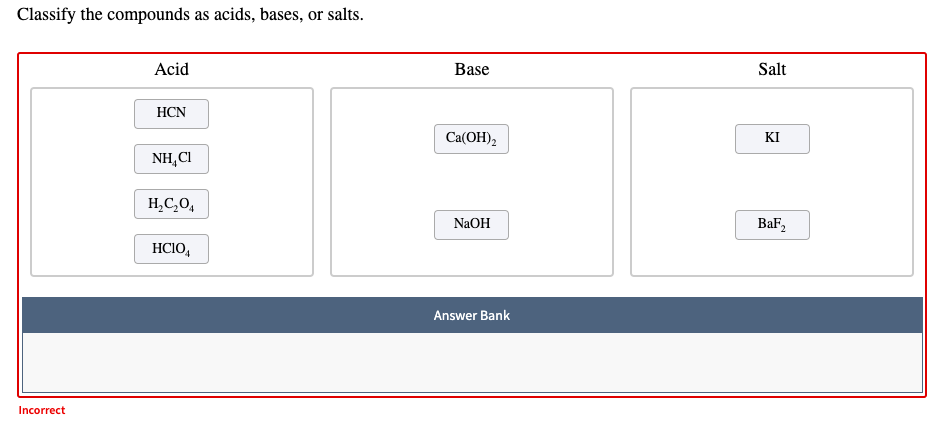
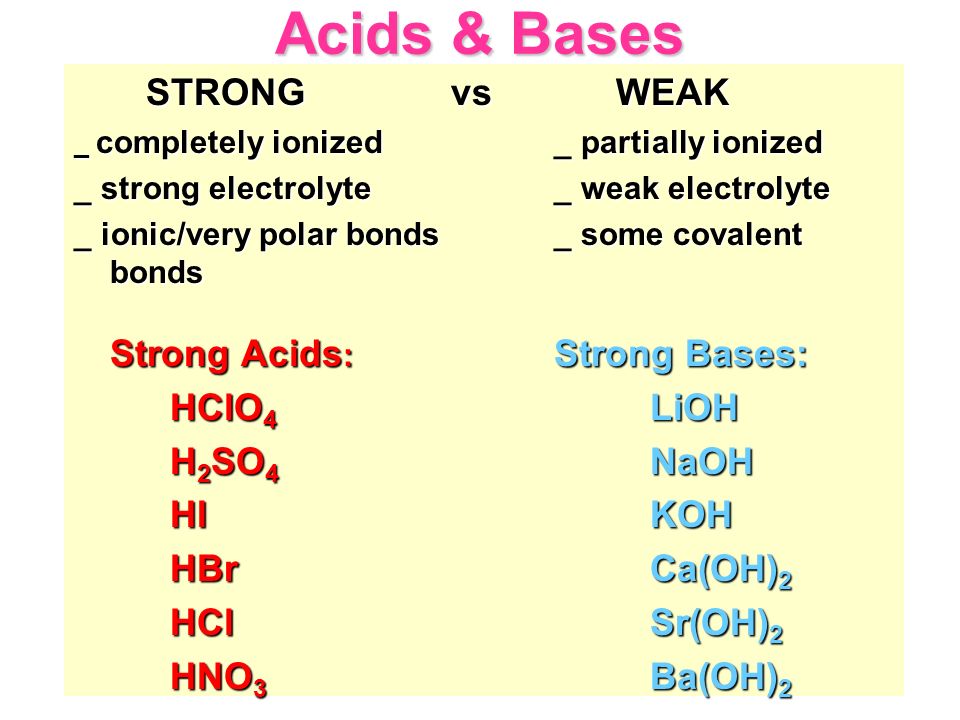
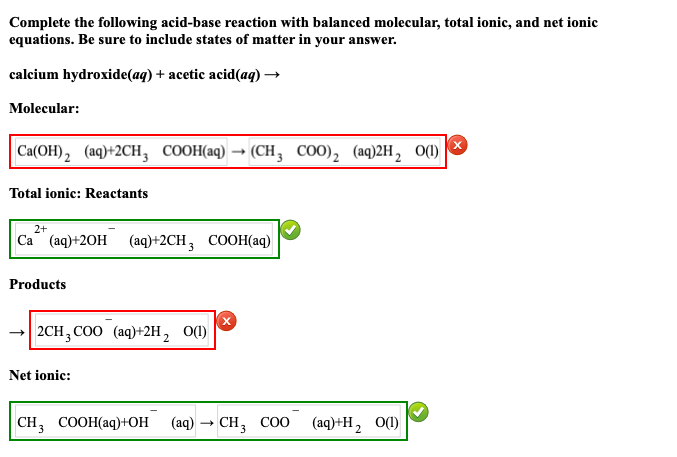
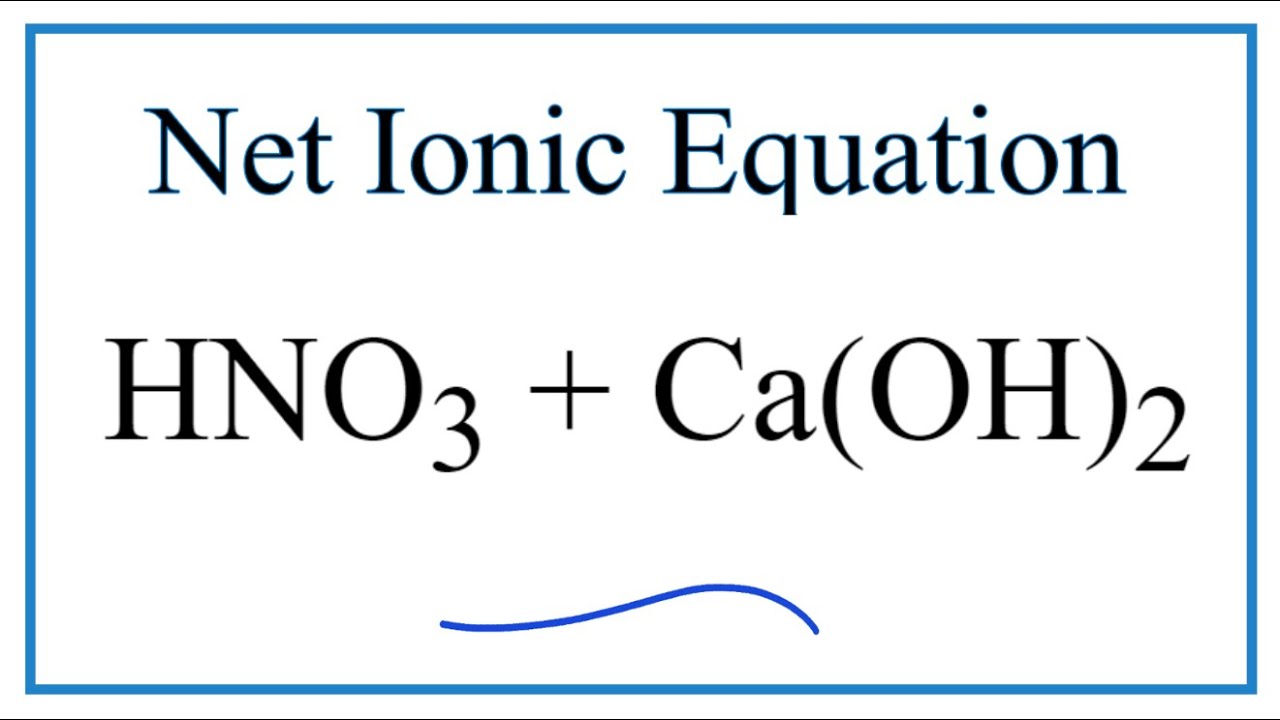
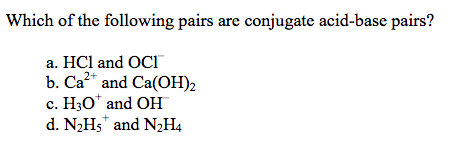
.PNG)
