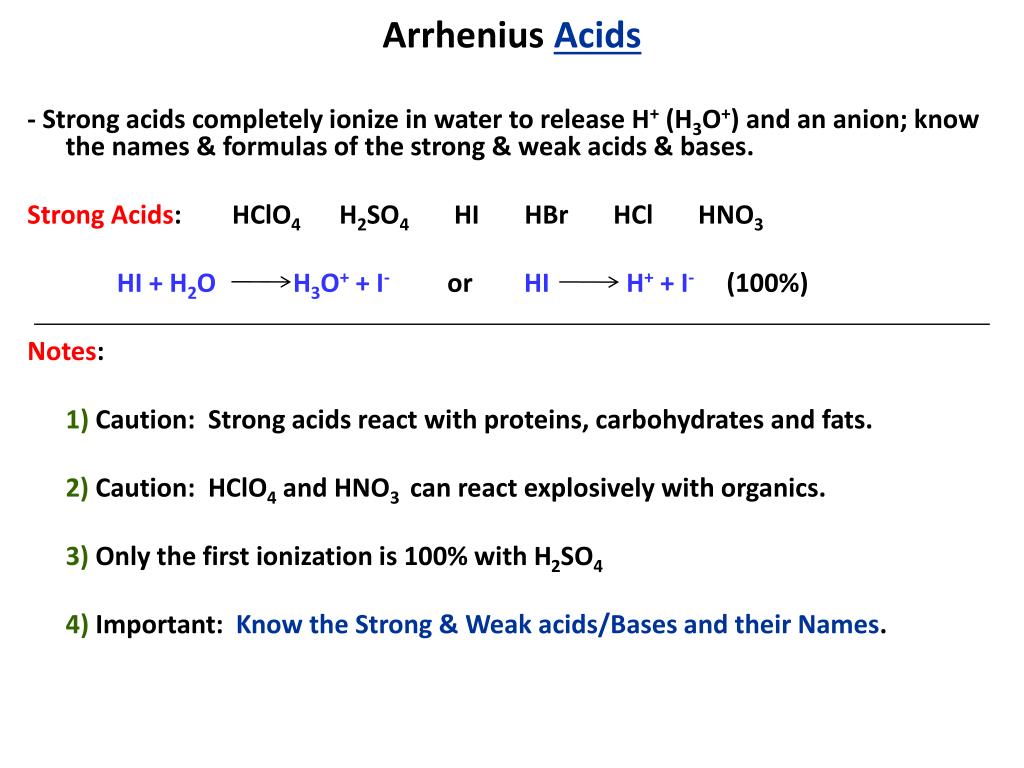
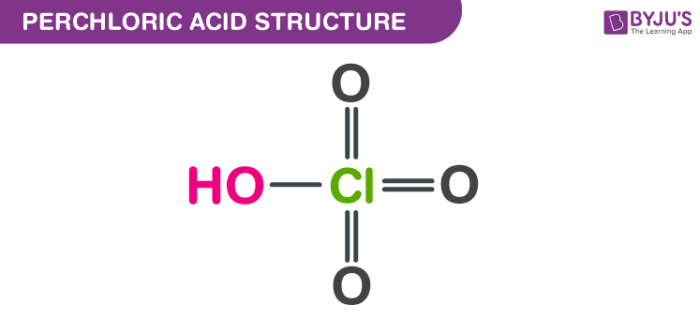
SOLVED: Enter a chemical equation for HClO4(aq) showing how it is an acid or a base according to the Arrhenius definition. Consider that strong acids and bases dissociate completely. Express your answer
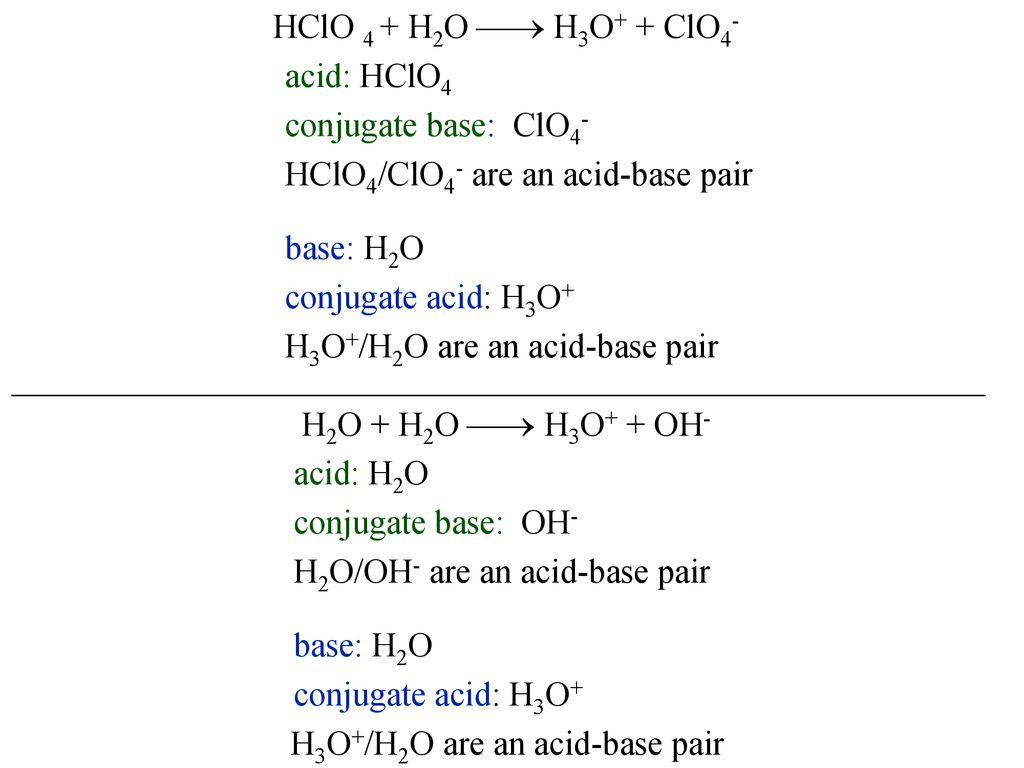
![In the following equation, what is acting as the Bronsted acid: [math]HClO_4 + H_2O\to ClO_4^- + H_3O^+[/math]? - Quora In the following equation, what is acting as the Bronsted acid: [math]HClO_4 + H_2O\to ClO_4^- + H_3O^+[/math]? - Quora](https://qph.cf2.quoracdn.net/main-qimg-db330d0184802e0ca001e7ca26960b0e.webp)
In the following equation, what is acting as the Bronsted acid: [math]HClO_4 + H_2O\to ClO_4^- + H_3O^+[/math]? - Quora
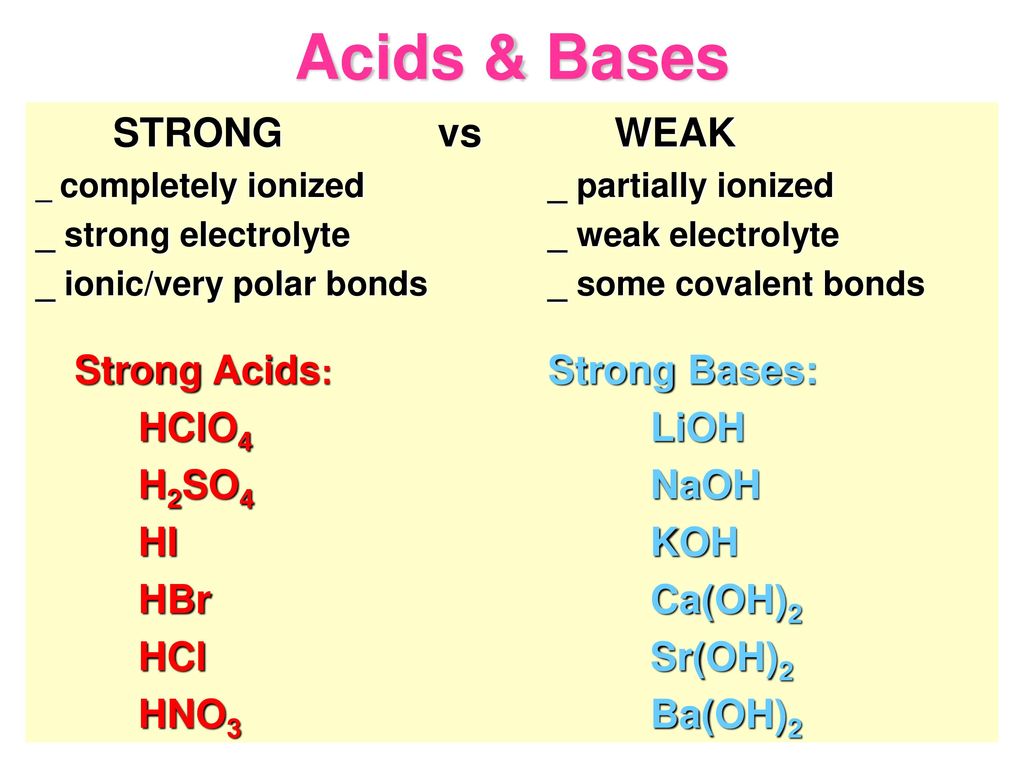
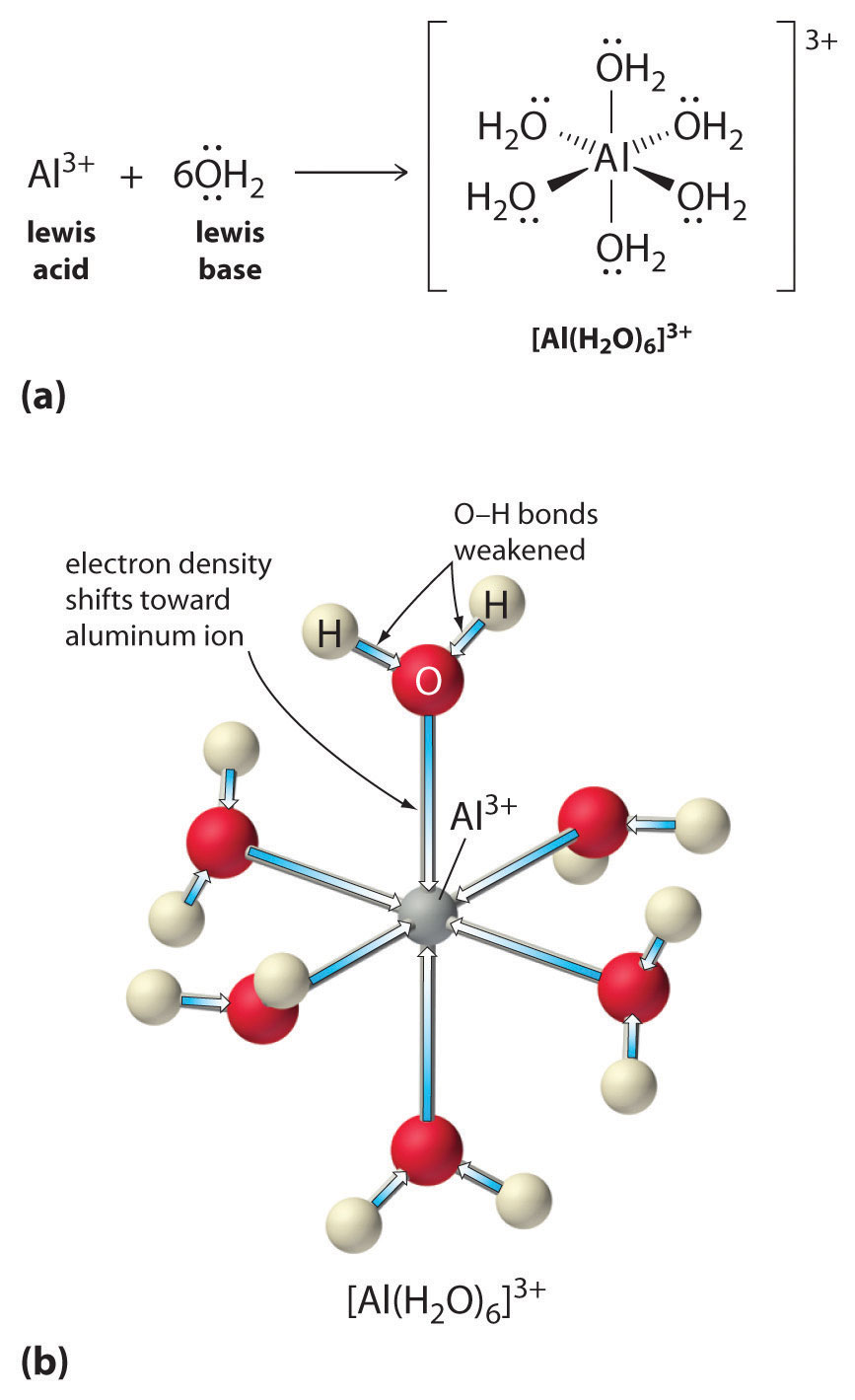
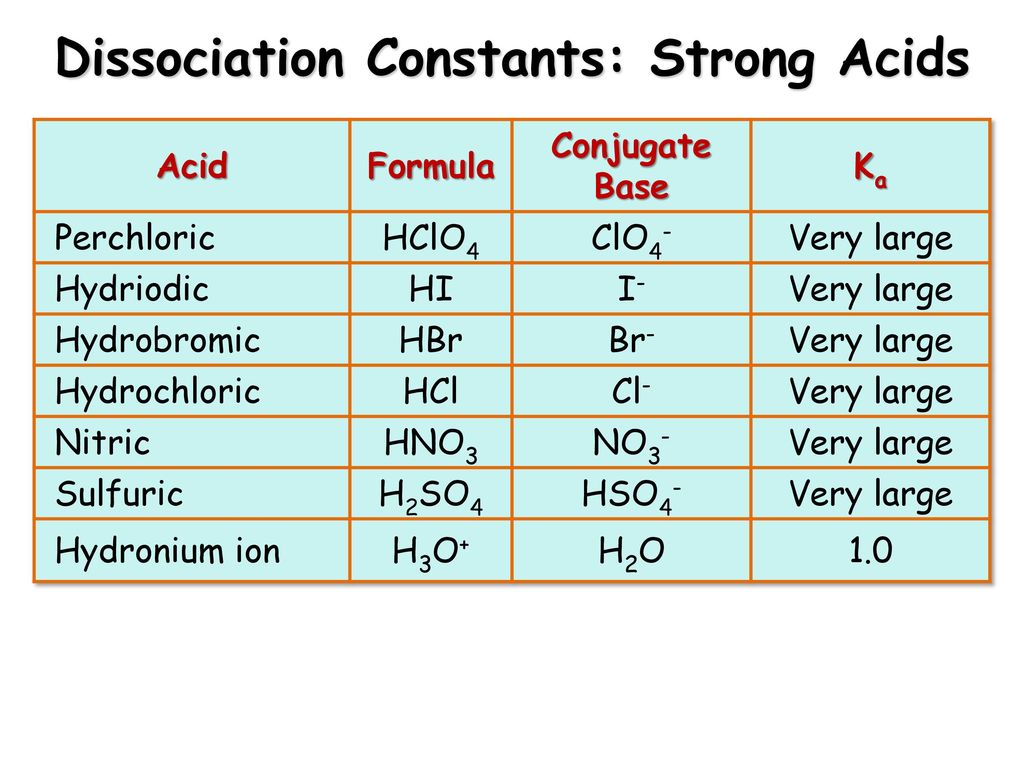
Acids & Bases Acids: acids are sour tasting Arrhenius acid: Any substance that, when dissolved in water, increases the concentration of hydronium. - ppt download
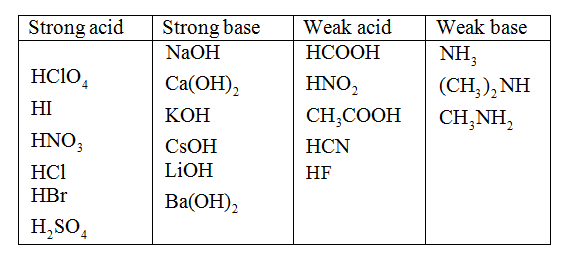
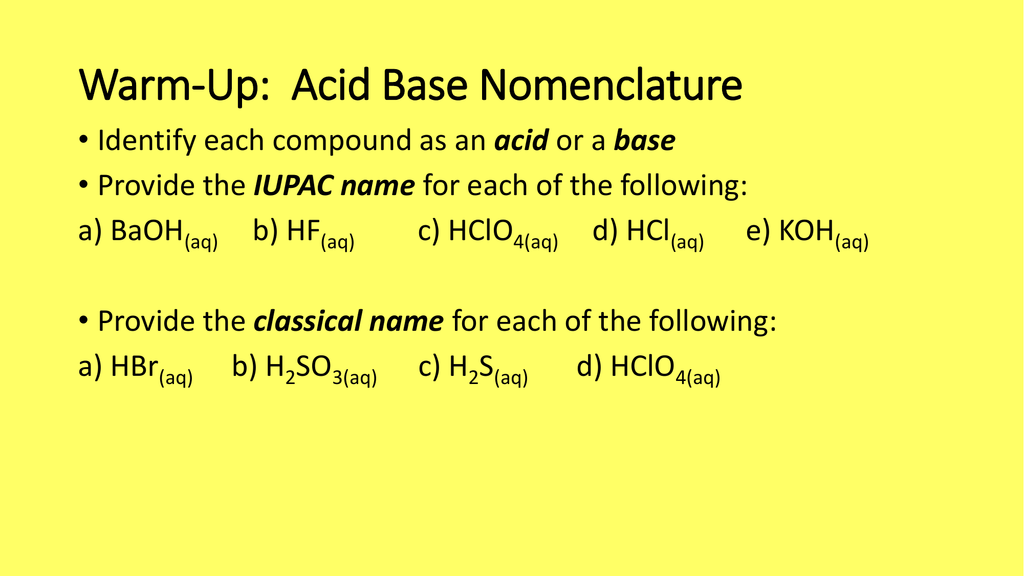
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum

What is meant by the conjugate acid-base pair? Find the conjugate acid//base for the following species: HNO(2), CN^(Θ), HClO(4), F^(Θ), overset(Θ)(O)H, CO(3)^(2-), and S^(2-)