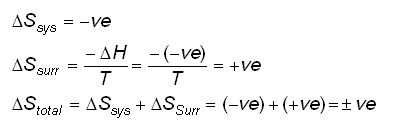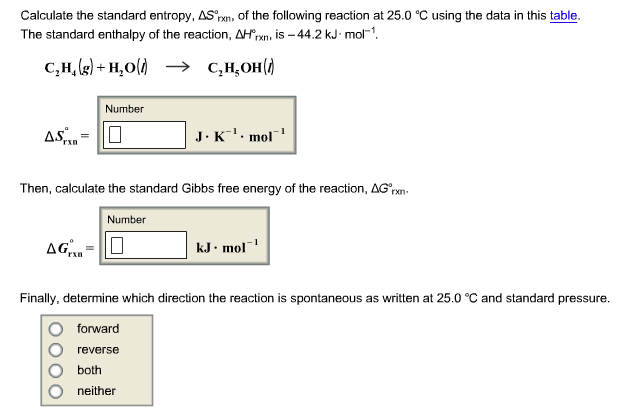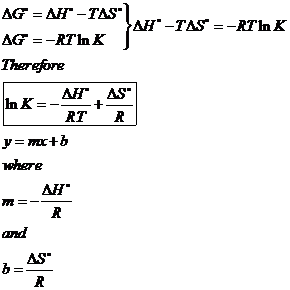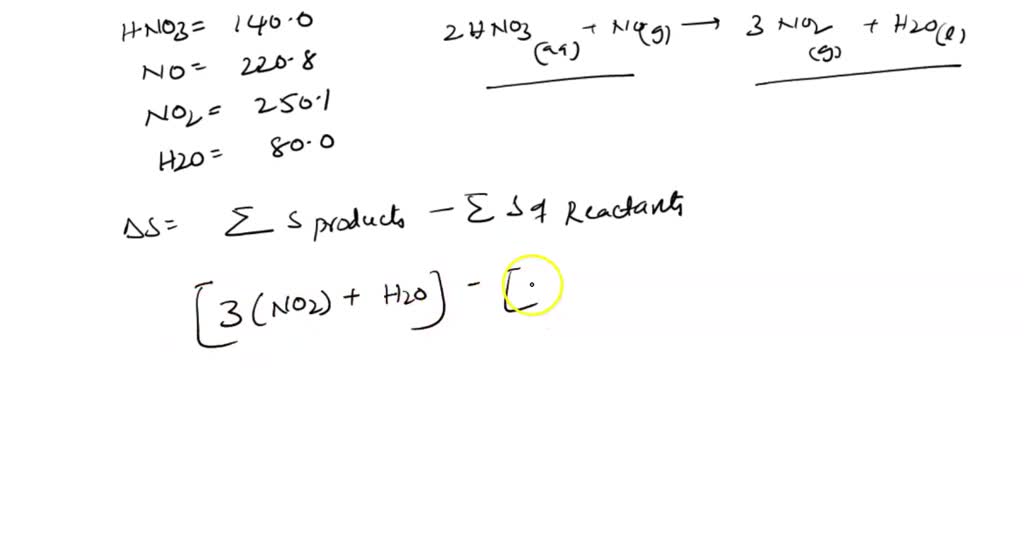
SOLVED: calculate delta S reaction (in J/mol *K) 2HNO3(aq)+NO(g) —> 3NO2(g)+H2O(l) delta S of... HNO3 = 140.0 NO= 220.8 NO2= 250.1 H2O= 80.0
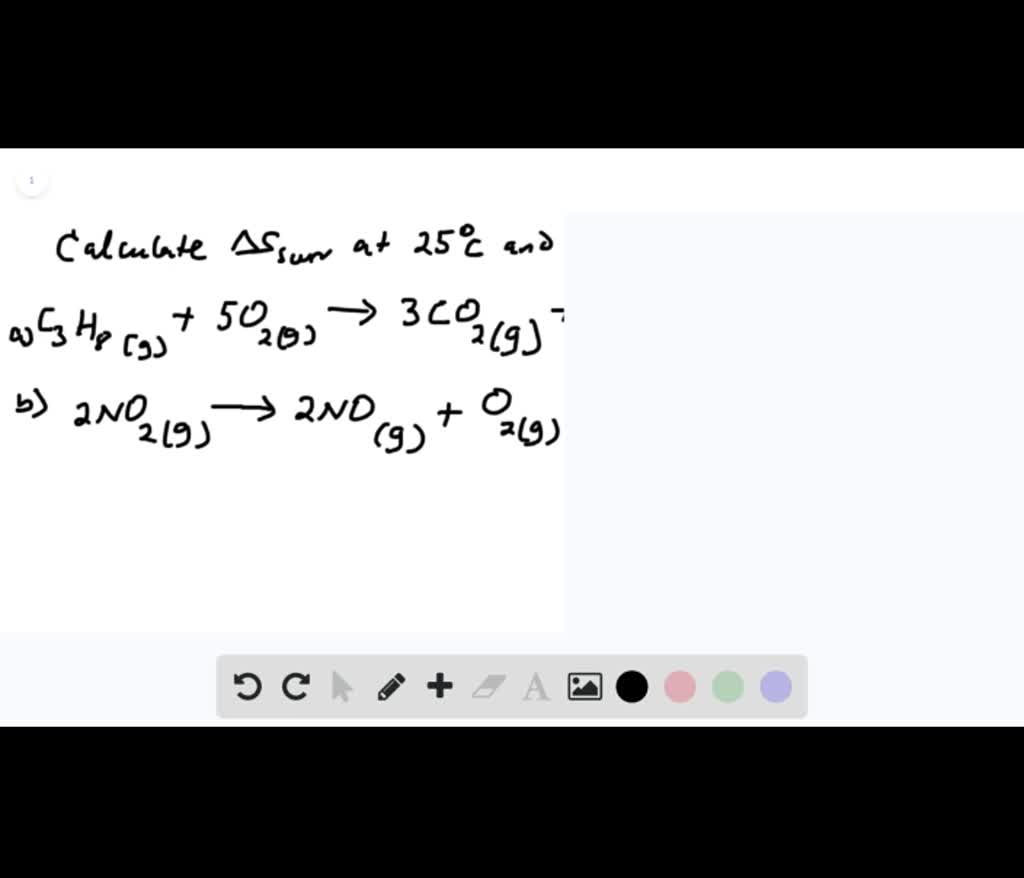
SOLVED:Calculate ΔS surr for the following reactions at 25^∘ C and 1 atm . a. C3 H8(g)+5 O2(g) ⟶3 CO2(g)+4 H2 O(l)ΔH^∘=-2221 kJ b. 2 NO2(g) ⟶2 NO(g)+O2(g) ΔH^ρ=112 kJ
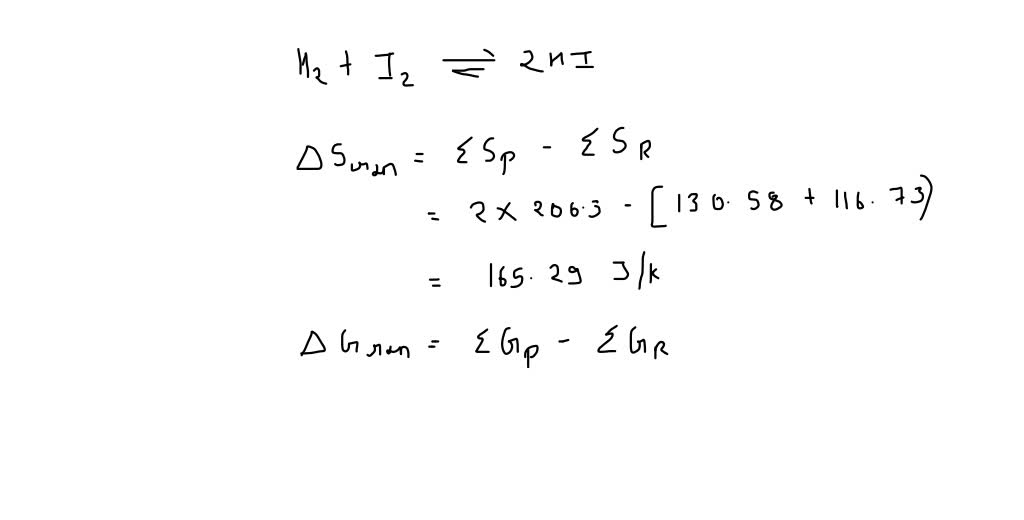
SOLVED: Use the values given below to Calculate delta S and delta H at 298K for the reaction H2 (g) + I2 (s) = 2HI (g) delta G delta S I2 (s)
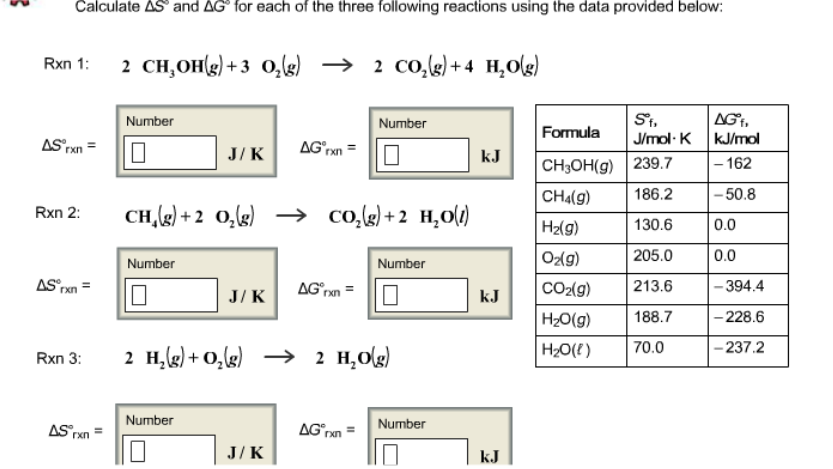
![Calculate deltaS and deltaG for each of t [{Image src='img236741055943655201772.jpg' alt='' caption=''}]he following 3 rxns using the data provided. Calculate delta S and delta G for each of the three | Homework.Study.com Calculate deltaS and deltaG for each of t [{Image src='img236741055943655201772.jpg' alt='' caption=''}]he following 3 rxns using the data provided. Calculate delta S and delta G for each of the three | Homework.Study.com](https://homework.study.com/cimages/multimages/16/img236741055943655201772.jpg)
Calculate deltaS and deltaG for each of t [{Image src='img236741055943655201772.jpg' alt='' caption=''}]he following 3 rxns using the data provided. Calculate delta S and delta G for each of the three | Homework.Study.com

SOLVED:Predict the sign of ΔS^∘ and then calculate ΔS^∘ for each of the following reactions. a. H2(g)+(1)/(2) O2(g) ⟶H2 O(l) b. 2 CH3 OH(g)+3 O2(g) ⟶2 CO2(g)+4 H2 O(g) c. HCl(g) ⟶H^+(a q)+
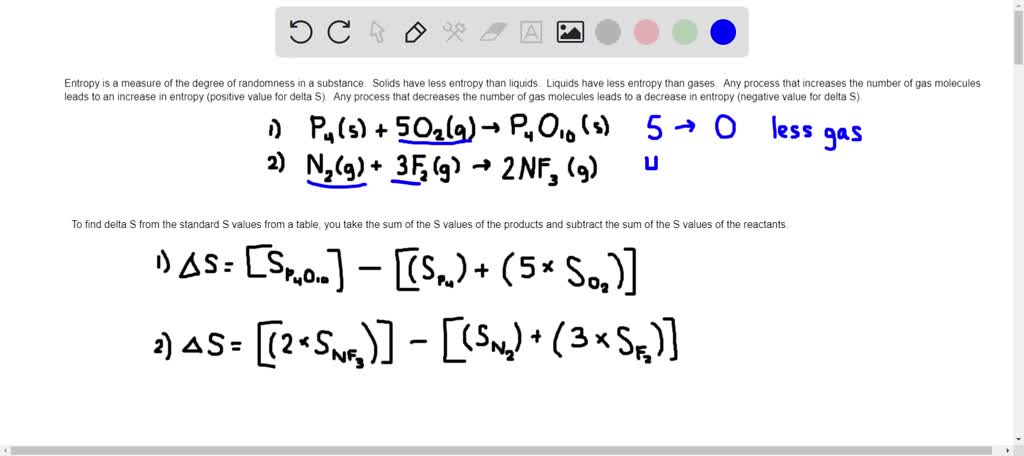
SOLVED: For each reaction (a) predict the sign and then using the standard entropy tables (b) find the value of delta S P4 (s) + 5O2 (g) -> P4O10 (s) N5 (g) +

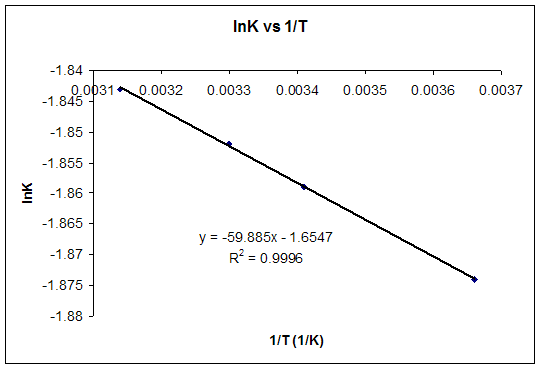
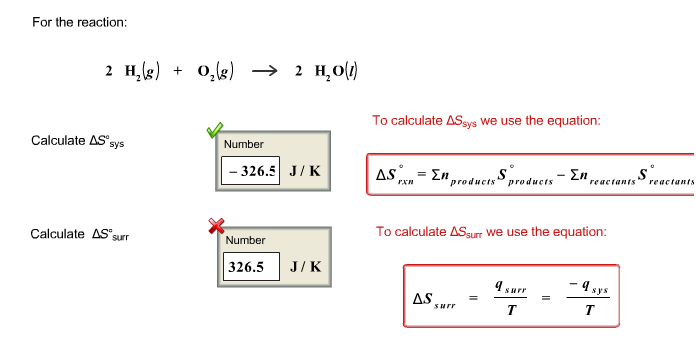
physical chemistry - Why doesn't Delta S total = 0 for this reversible process? - Chemistry Stack Exchange