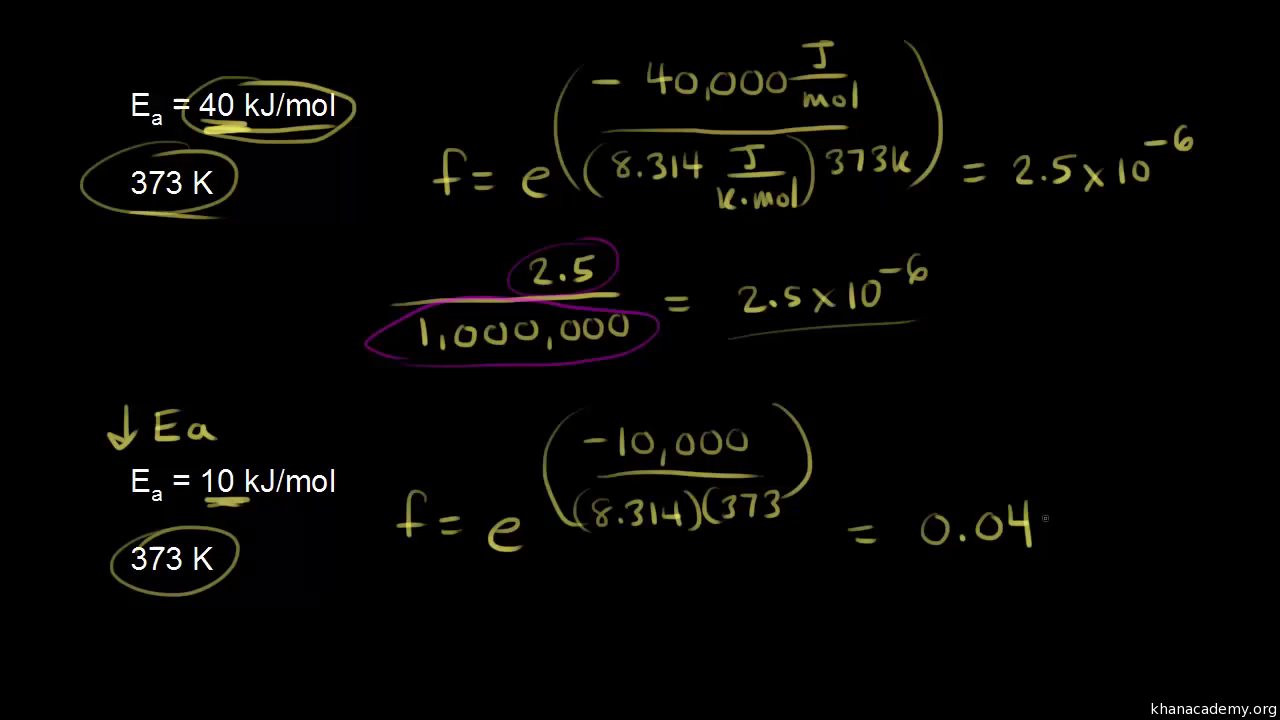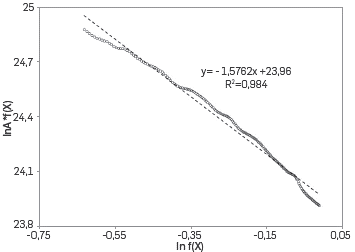
METHODOLOGY FOR CALCULATING THE PRE-EXPONENTIAL FACTOR USING THE ISOCONVERSIONAL PRINCIPLE FOR THE NUMERICAL SIMULATION OF THE AIR INJECTION PROCESS

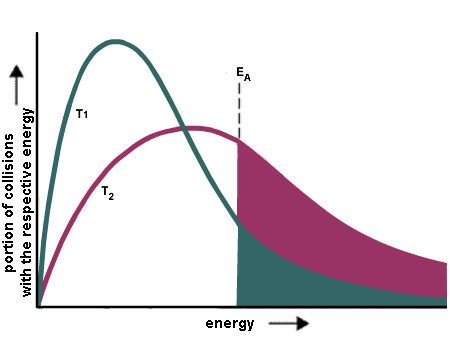
Collision Theory and activation energy|Pre exponential factor Arrhenius equation|Rank Booster - YouTube

Chapter 25 The Rates of chemical reactions. Contents Empirical chemical kinetics 25.1 Experimental techniques 25.2 The rates of reactions 25.3 Integrated. - ppt download

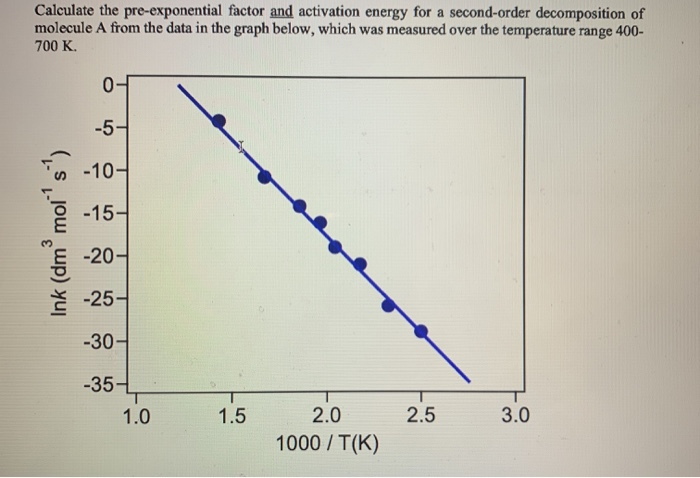
The rate constant for the decomposition of hydrocarbons is 2.418 × 10^-5s^-1 at 546 K . If the energy of activation is 179.9 kJ/mol , what will be the value of pre - exponential factor?

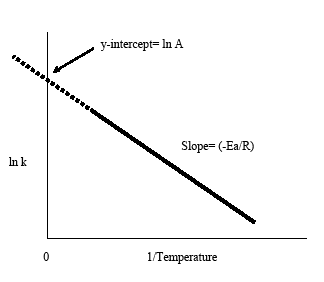
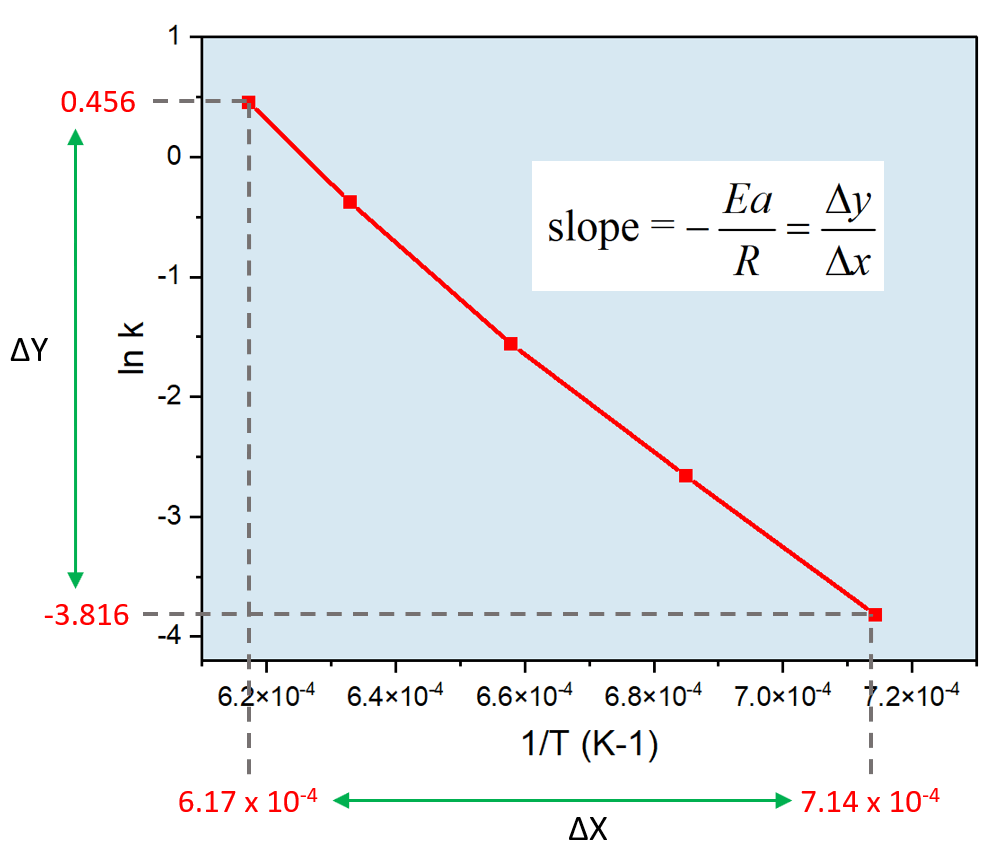
16.3.2 Determine activation energy (Ea) values from the Arrhenius equation by a graphical method. - YouTube
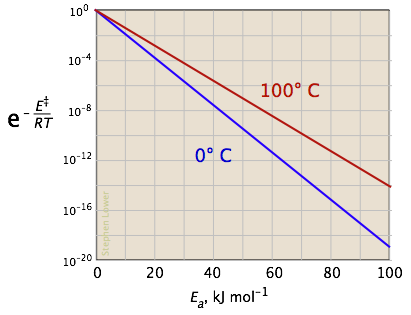
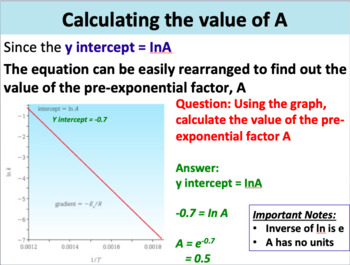
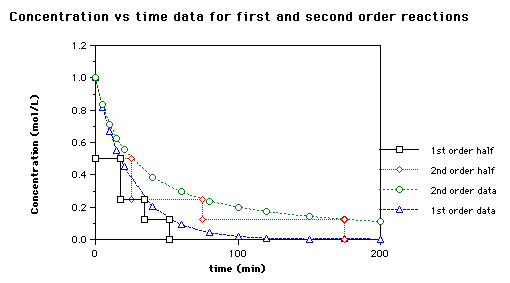
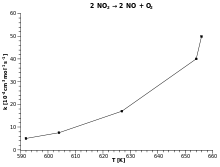
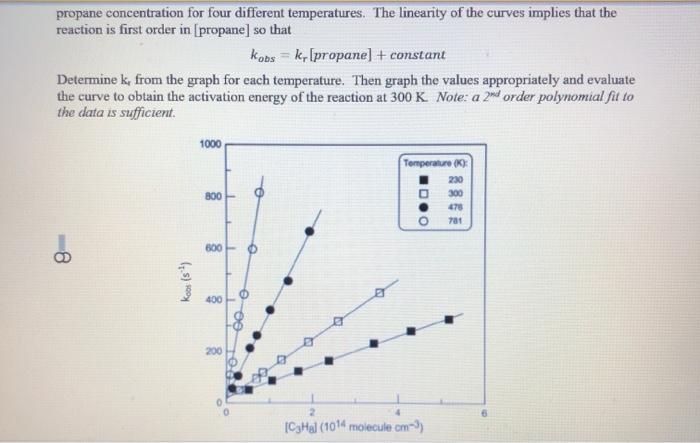
Chemical Kinetics Goal: Our goal in this activity is to explore some applications of chemical kinetics. We begin by thinking c