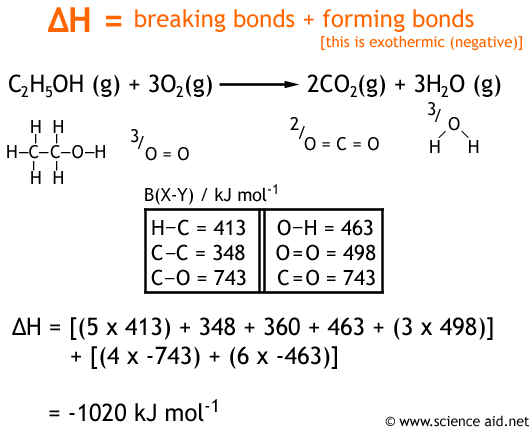
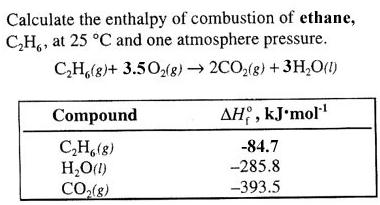
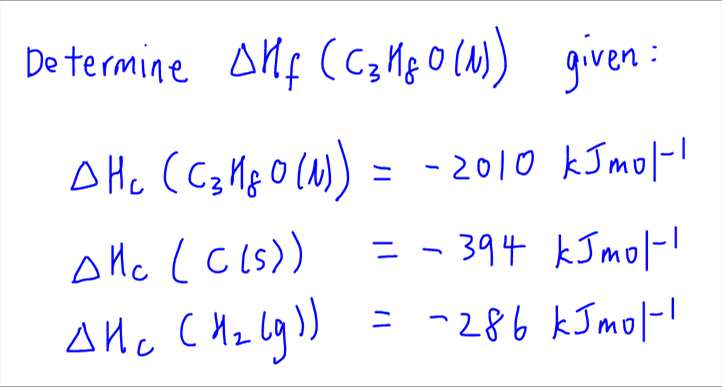Calculate the enthalpy of combustion of ethylene at 1 atm pressure and 298 K, if enthalpy of formation of CO2(g) , H2O(I) and C2H4(g) are - 394, - 242 and - 52 kJ respectively.
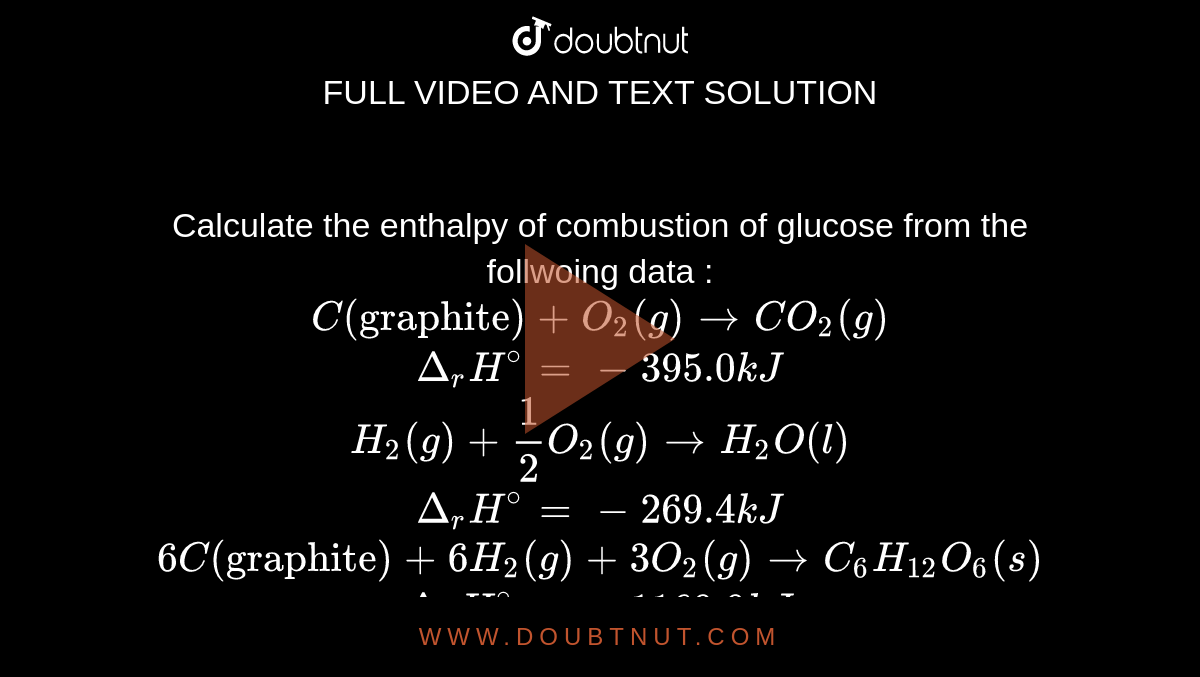
Calculate the enthalpy of combustion of glucose from the follwoing data : C("graphite") +O(2)(g) to CO(2)(g) Delta(r)H^(@) = -395.0 kJ H(2)(g) + 1/2 O(2)(g) to H(2)O(l) Delta(r)H^(@) = - 269.4 kJ 6C("graphite") +

⚗️Use the information from the diagram to calculate the enthalpy of combustion for methane.(IGNORE MY - Brainly.com

Chemical Energetics: Experimental Method to Determine Enthalpy Change of Combustion - A-Level H2 Chemistry Tuition by 10 Year Series Author

Question Video: Calculating the Enthalpy Change for the Reaction between Phenol and Diatomic Hydrogen Using Standard Enthalpies of Combustion | Nagwa