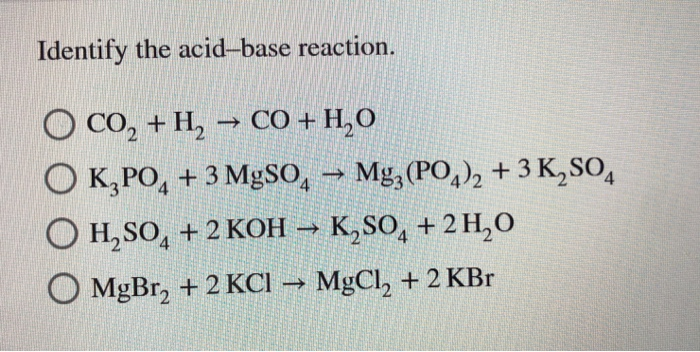The Importance of Acid–Base Equilibria in Bicarbonate Electrolytes for CO2 Electrochemical Reduction and CO Reoxidation Studied on Au(hkl) Electrodes | Langmuir
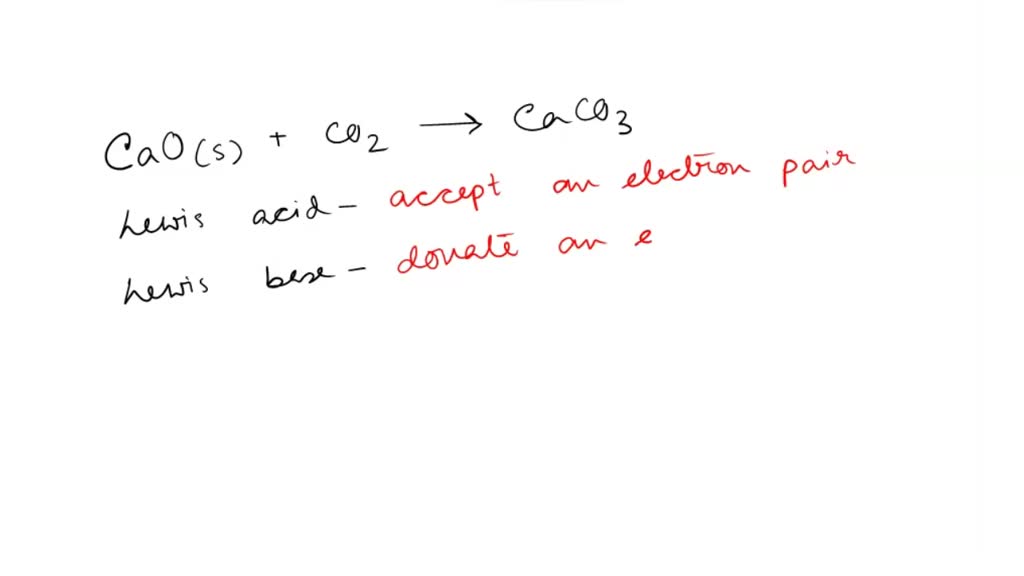
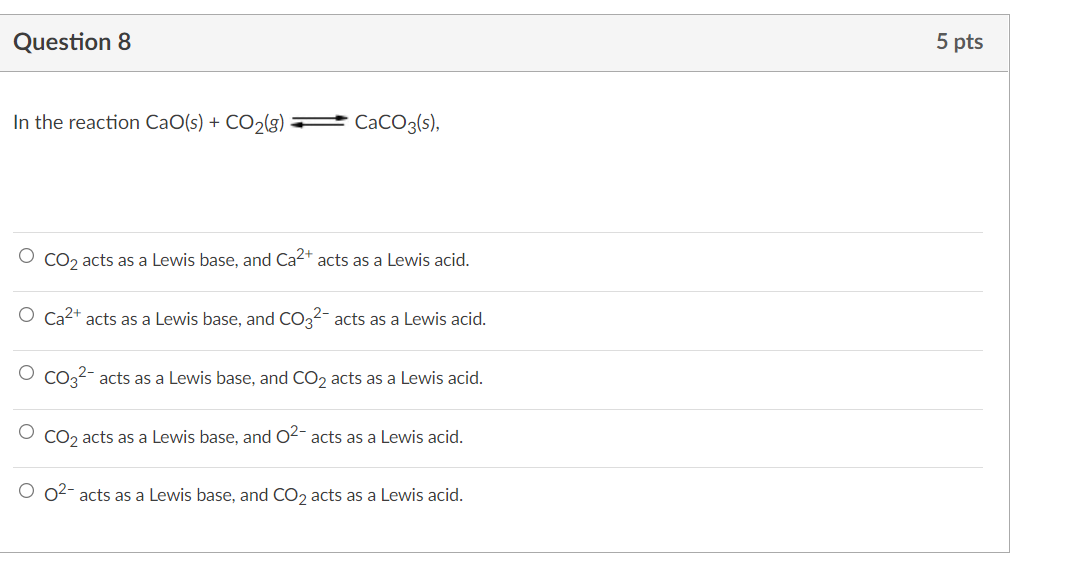
SOLVED: QUESTION 1 In the reaction: CaO(s) + CO2(g) â†' CaCO3(s), Ca2+ acts as a Lewis acid and CO32- acts as a Lewis base. CO2 acts as a Lewis acid and CO32-
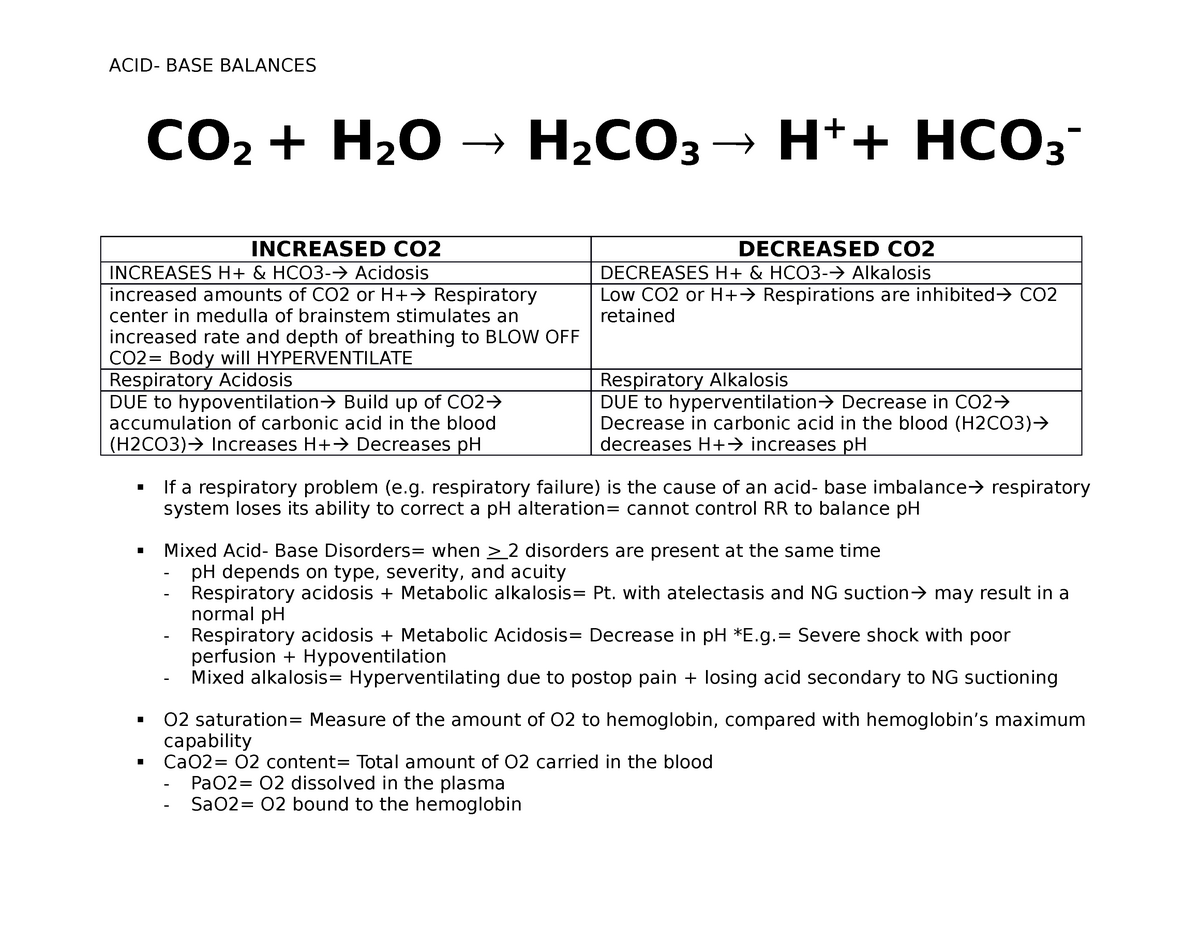
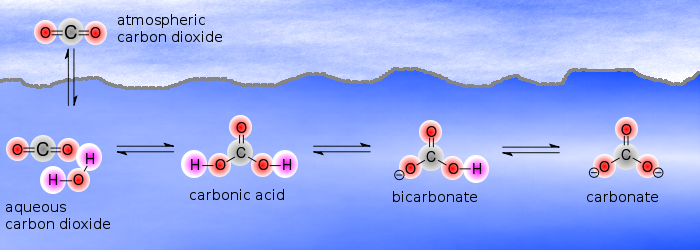
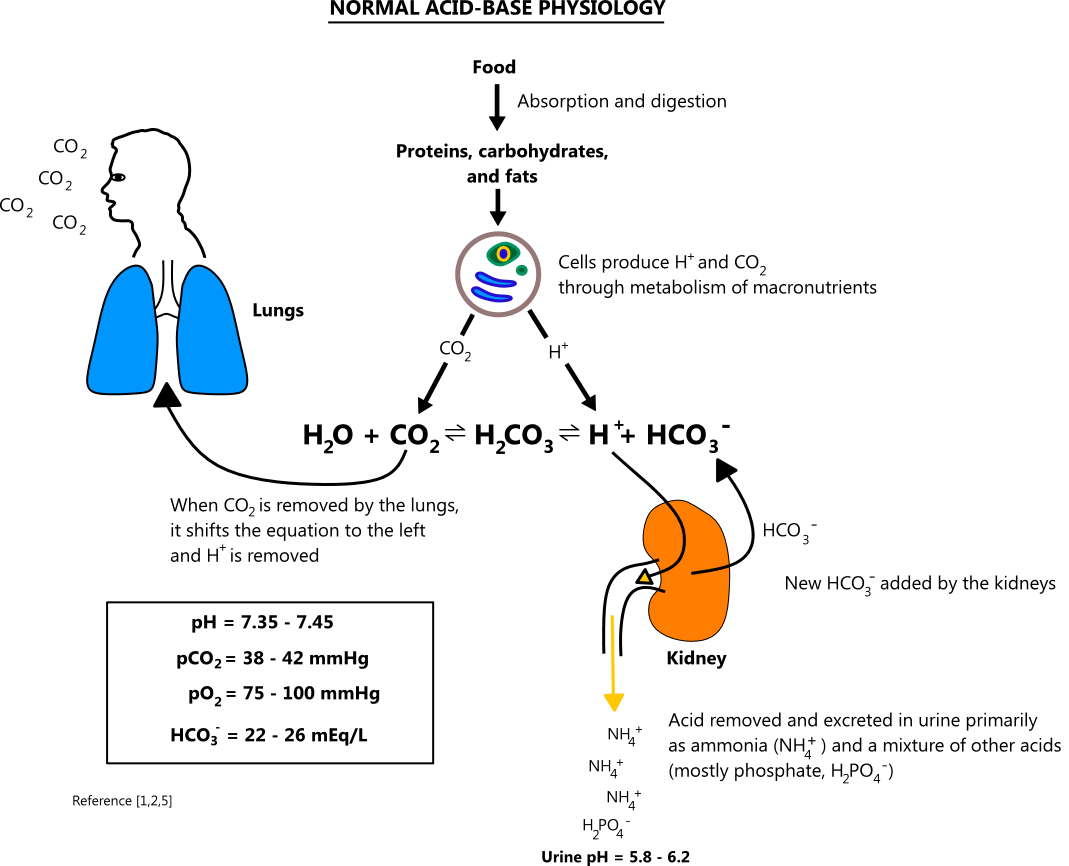
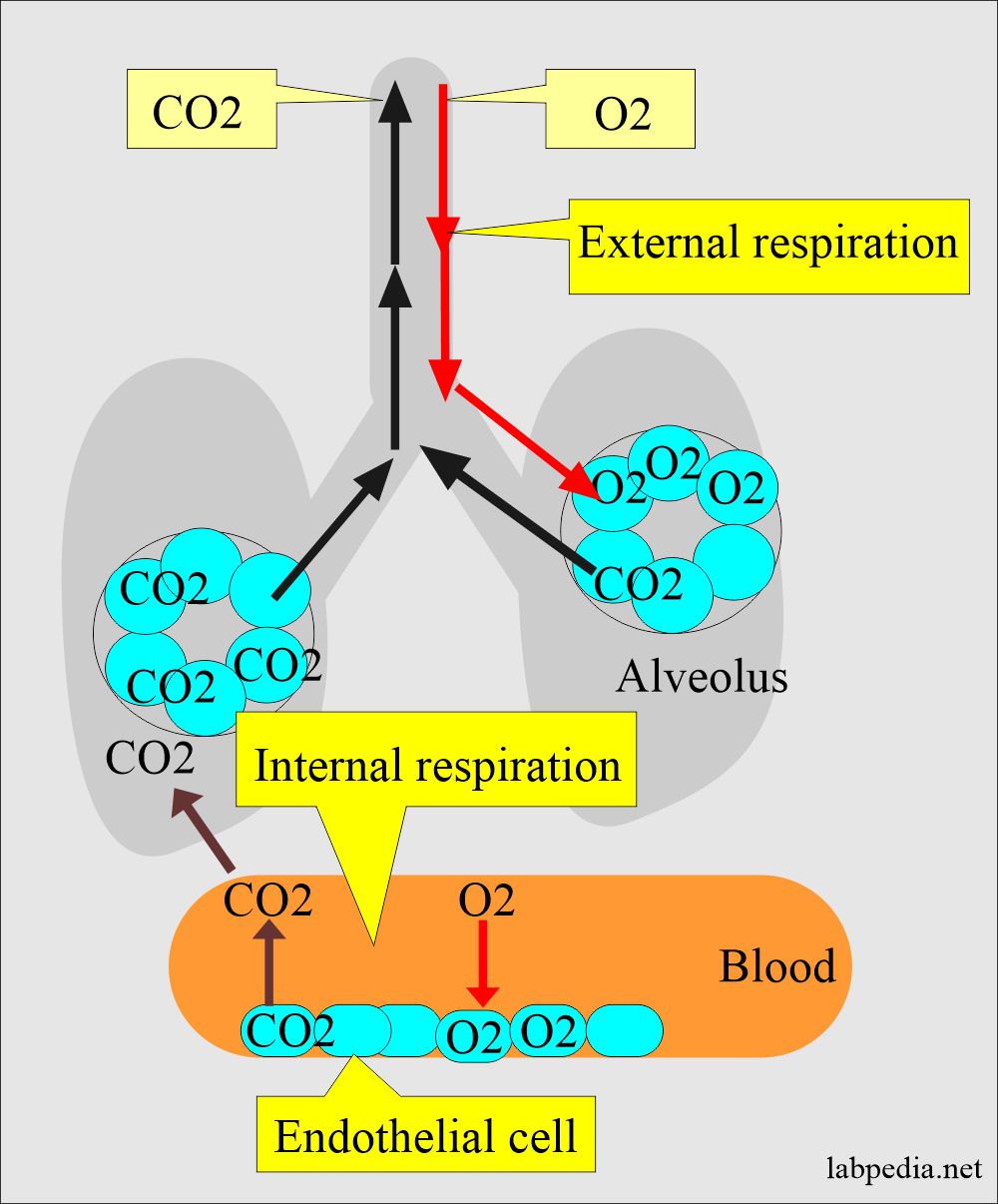
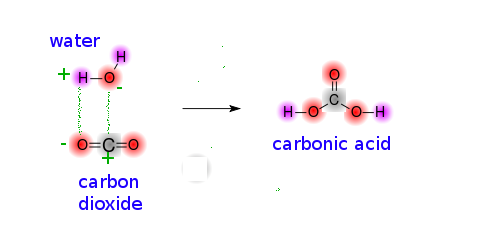
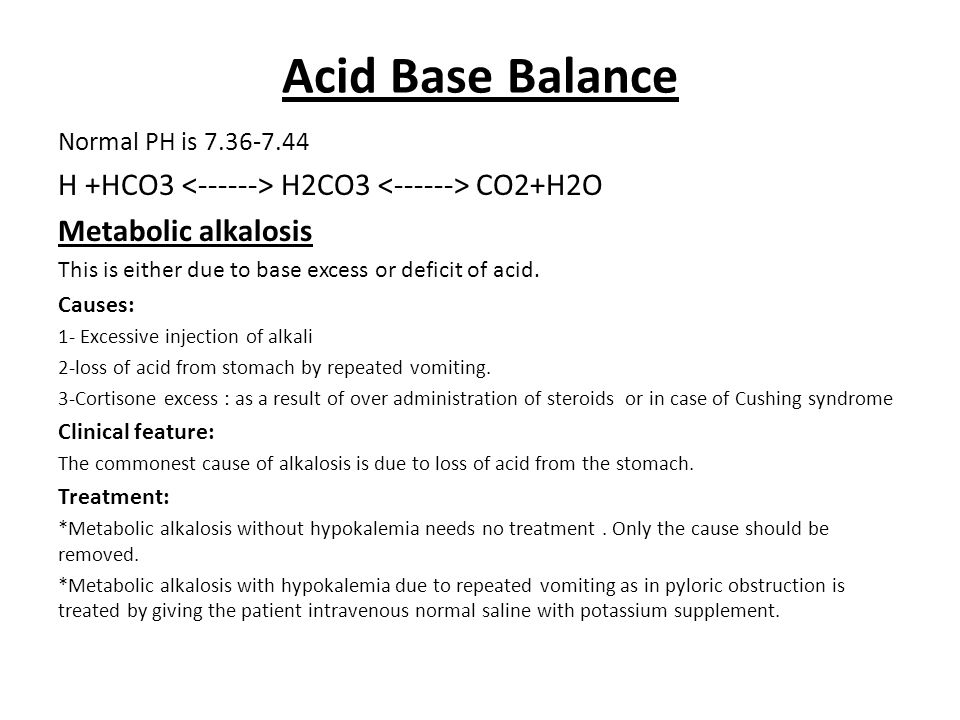
Acid- Base Imbalances - Lecture notes 1 - CO 2 + H 2 O ® H 2 CO 3 ® H + + HCO 3 – INCREASED CO2 - Studocu