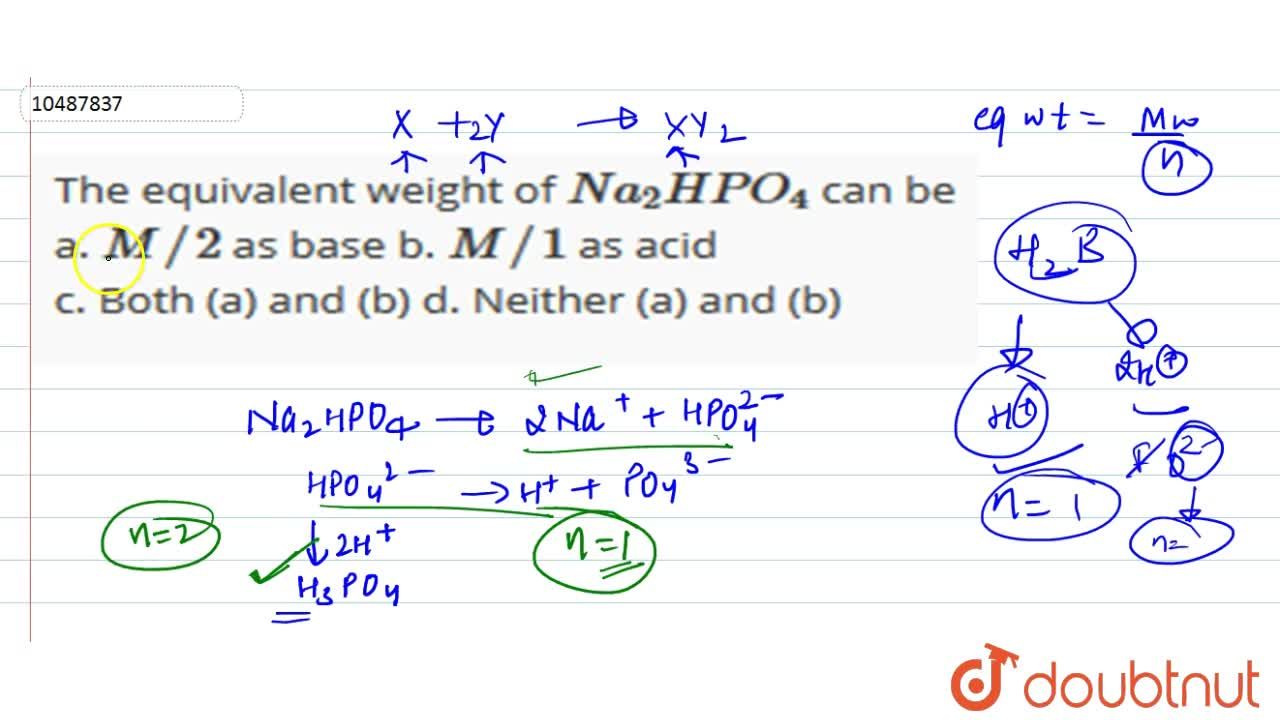
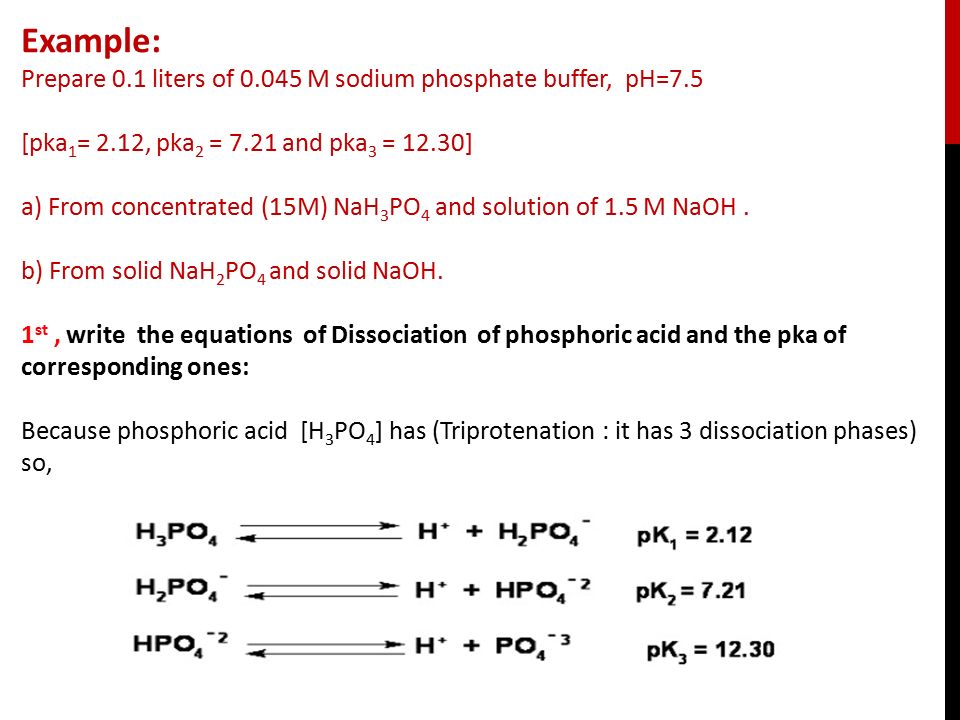
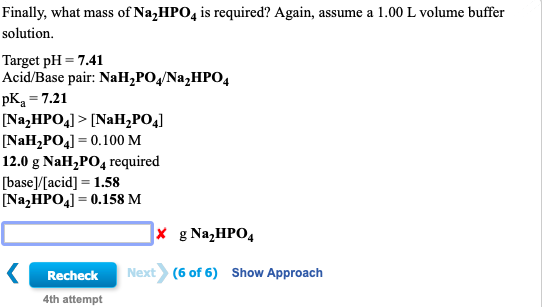
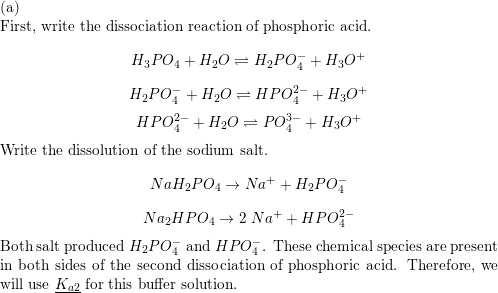Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142

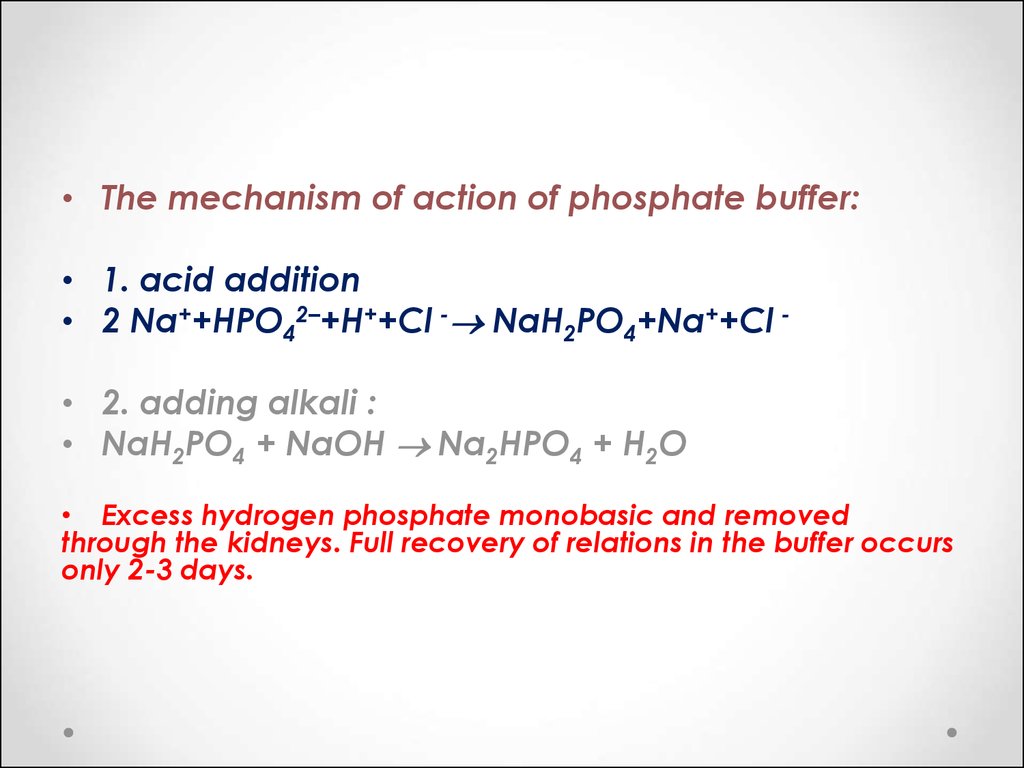
A buffer solution 0.04 M in Na2HPO4 and 0.02 M in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli - mole of the organic compound RNHOH is carried out in 100

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

If 2.5 moles each of H3PO4,NaH2PO4,Na2HPO4 and Na3PO4 are mixed together to form an aqueous solution, then the resulting pH is:Given values of Ka are: Ka1 = 10^-3 Ka2 = 10^-7 Ka3 = 10^-13
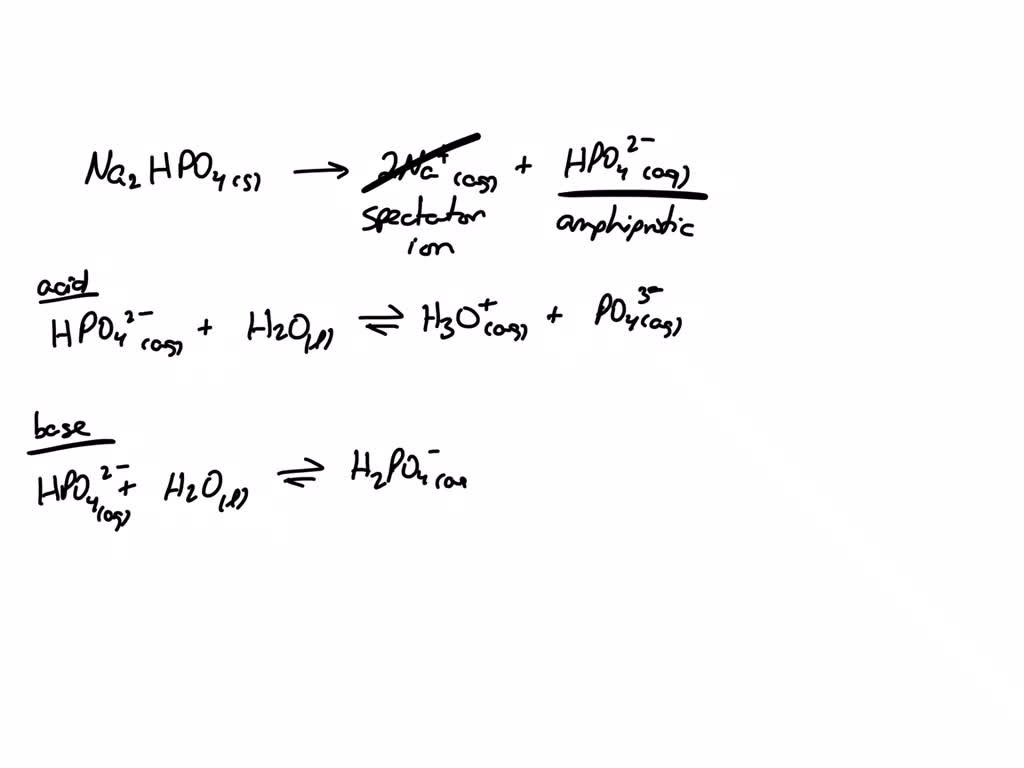
SOLVED: Predict whether the salt Na2HPO4 forms an acidic solution or a basic solution when dissolved in water.